Separation by a membrane is achieved by creating a boundary between different bulk gas or liquid mixtures. As different solvents and solutes flow through a membrane at different rates, separation is achieved.
Here, we will focus on three filtration techniques: microfiltration (MF), ultrafiltration (UF) and nanofiltration (NF). These processes are characterized by the size of the particle that can be separated by the membrane, as illustrated in the figure. Each membrane type is best suited for unique applications and is designed with the module and material that will allow the best separation.
Flow through a membrane is characterized as either tangential flow filtration (TFF), where the feed stream flows at a velocity vector normal to the membrane surface, or normal flow filtration (NFF), where the stream flows tangent to the membrane surface. The flow pattern is dependent on the type of module utilized. NFF modules include: cartridges, stacked disks and flat sheets. TFF modules include: plateandframe (cassettes), hollow fibers, tubes, monoliths, spirals and vortex flow.
Microfiltration
MF separates particles from true solutions. This technique is able to separate particles from about 0.1 to as high as 10 μm. As can be seen from the figure, large, soluble macromolecules, bacteria and other microorganisms can be retained by MF membranes.
Membrane materials
MF membranes have the largest pore openings of any other membrane. Typically, they can be classified as having tortuous or capillary pores.
From solids. When membranes are made by sintering or agglomeration of microparticles, pores are formed by the interstices between solid particles. Common materials include: metal, metal oxide, graphite, ceramic and polymer.
Ceramic. These membranes are typically created by the sol-gel process, which is the successive deposition of smaller ceramic precursor spheres, followed by firing to form multitube monoliths.
Track etched. A polymer film is exposed to a collimated beam of radiation that breaks chemical bonds in the polymer chains. The film is then etched in a bath that selectively attacks the damaged polymer, a technique that produces a film with photogenic pores.
Chemical phase inversion. A solution of a concentrated polymer in solvent is spread into a thin film, then precipitated through the slow addition of a nonsolvent to produce tortuous pores.
Thermal phase inversion. A solution of polymer in a poor solvent is prepared at an elevated temperature. After being formed into its final shaped, the temperature is dropped and the polymer precipitates, and the solvent is washed out.
Streched polymers. Semicrystalline polymers, which are stretched perpendicular to the axis of crystallite orientation, fracture in such a way that reproducible microchannels are made.
Membrane modules
Many conventional designs are used in MF, including cartridge-filter housing, plate-andframe-type devices, capillary bundles, tubular membranes, spiral-wound modules and belt filters. Ceramic MF membranes are available as flat sheet, single tubes, disc, and other forms, primarily for lab use. Finally, cassettes are two different cross-flow membrane devices.
Ultrafiltration
UF membranes, with pore sizes ranging from about 1 to 100 nm in diameter, employ pressure driving forces of 0.2–1.0 MPa. This technique drives liquid solvents and small solutes through the membrane, while retaining larger particles, like large dissolved molecules, colloids and suspended solids.
Membrane materials
UF membranes are typically made of polymeric structures, such as polyethersulfone, regenerated cellulose, polysulfone, polyamide, polyacrylonitrile or various fluropolymers. They are formed by immersion casting on a web or as a composite on an MF membrane. Membrane selection is based on molecular-weight rating for high yields, chemical and mechanical robustness during product processing and Clean In Place, and process flux for sizing and costing.
Membrane modules
Modules include cassettes, spirals, hollow fibers, tubes, flat sheets, and inorganic monoliths. These primarily operate in TFF to increase flux by reducing plugging. For virus removal and water treatment, however, NFF operation is run with cartridge and hollow fiber modules.
Nanofiltration
NF, sometimes referred to as “loose RO (reverse osmosis),” utilizes a driving force of 0.3 to 10.5 MPa to drive liquid solvents through the membrane while retaining small solutes of about 10 to 100 nm in diameter.
NF membranes are different from the membranes previously discussed, because they are usually charged, utilizing ion repulsion as a major method of charged-species rejection.
They have 20–80% NaCl retention and retain > 200–1,000 Daltons of neutral organics, with a low retention of dissolved gases.
Neutral or undissociated solutes have a lower retention than charged or dissociated solutes.
Membrane materials
Cellulose polymers. These are formed by immersion casting of 30–40% polymer lacquers, which can include cellulose acetate, triacetate and acetate-butyrate, on a web immersed in water.
Thin film composites. Formed by interfacial polymerization, TFCs involve coating a microporous membrane substrate with an aqueous prepolymer solution, then immersing it in a water-immiscible solvent containing a reactant.
Crosslinked polyetherurea. Some of these membranes feature NaCl retention and water permeability.
Membrane modules
NF membrane modules are available in spiral, hollow fiber, tubular, and plate-andframe formats. Spirals are most common, as they have low feed-side pressure props, are less prone to clogging, are easily cleaned, are mechanically robust, and are most economical.
References
1. “Perry’s Chemical Engineers’ Handbook,” 8th ed. McGraw Hill, New York, 2008.
2. Seidel, A., ed. in chief, “Separation Technology,” second edition, John Wiley and Sons, Inc., New Jersey, 2008.
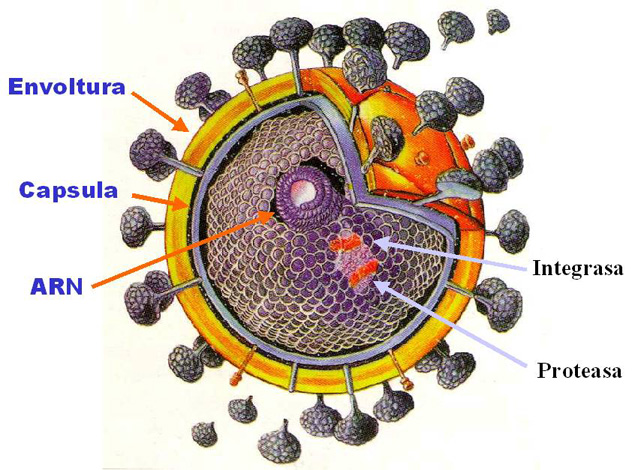



















































 El principal componente químico de los granos de sorgo es el almidón, que puede variar entre un 70 y un 80% de la materia seca del grano. Los gránulos de almidón están compuestos por dos moléculas principales (amilosa y amilopectina), cuya proporción determina las propiedades reológicas y adhesivas del producto. Las moléculas de amilasa permiten una buena incorporación del adhesivo dentro del papel por la forma de su estructura molecular, mientras que altos porcentajes de esta molécula facilitan una mayor velocidad de pegado inicial; proceso conocido en la industria bajo el nombre de green bond. Al sorgo se lo puede clasificar además por el contenido de taninos. Éstos son compuestos cuya propiedad principal es la de actuar como antioxidante y antibacteriano.
El principal componente químico de los granos de sorgo es el almidón, que puede variar entre un 70 y un 80% de la materia seca del grano. Los gránulos de almidón están compuestos por dos moléculas principales (amilosa y amilopectina), cuya proporción determina las propiedades reológicas y adhesivas del producto. Las moléculas de amilasa permiten una buena incorporación del adhesivo dentro del papel por la forma de su estructura molecular, mientras que altos porcentajes de esta molécula facilitan una mayor velocidad de pegado inicial; proceso conocido en la industria bajo el nombre de green bond. Al sorgo se lo puede clasificar además por el contenido de taninos. Éstos son compuestos cuya propiedad principal es la de actuar como antioxidante y antibacteriano.







 Las metodologías tradicionales han sido el hisopado de superficies y la exposición de placas de cultivo al ambiente a fin de producir un conteo por sedimento, esta práctica se ha mejorado a través del tiempo, pero nunca se ha logrado eliminar el error que introduce este tipo de medición en los resultados de los ensayos.
Las metodologías tradicionales han sido el hisopado de superficies y la exposición de placas de cultivo al ambiente a fin de producir un conteo por sedimento, esta práctica se ha mejorado a través del tiempo, pero nunca se ha logrado eliminar el error que introduce este tipo de medición en los resultados de los ensayos. Lo recomendable en estos casos es asesorarse con especialistas en sistemas de tratamiento de aire a fin de dar solución definitiva al problema. Actualmente existen unidades móviles de filtración de aire (99,99% eficiencia en partículas de 0,3 um) con alto caudal de trabajo y de fabricación nacional, que colaboran de manera local a mejorar la calidad de aire interior, sin requerir modificaciones en el sistema de ventilación general.
Lo recomendable en estos casos es asesorarse con especialistas en sistemas de tratamiento de aire a fin de dar solución definitiva al problema. Actualmente existen unidades móviles de filtración de aire (99,99% eficiencia en partículas de 0,3 um) con alto caudal de trabajo y de fabricación nacional, que colaboran de manera local a mejorar la calidad de aire interior, sin requerir modificaciones en el sistema de ventilación general.












 Para continuar las tareas de finalización de Atucha II Nucleoeléctrica Argentina SA ha formulado un detallado plan de trabajos que incluye todas las actividades de ingeniería, construcción y montaje pertinentes. Los materiales y equipos necesarios se encuentran ya almacenados en la obra.
Para continuar las tareas de finalización de Atucha II Nucleoeléctrica Argentina SA ha formulado un detallado plan de trabajos que incluye todas las actividades de ingeniería, construcción y montaje pertinentes. Los materiales y equipos necesarios se encuentran ya almacenados en la obra.








